 Last week’s specimen was a Lower Carboniferous fossiliferous siderite concretion from an unknown location, but likely from the Wooster area. It was donated to the department by Emeritus Geology Professor Sam Root. The concretion has beautiful crinoids preserved in it, including several stems of at least two types and three calices (crowns or heads).
Last week’s specimen was a Lower Carboniferous fossiliferous siderite concretion from an unknown location, but likely from the Wooster area. It was donated to the department by Emeritus Geology Professor Sam Root. The concretion has beautiful crinoids preserved in it, including several stems of at least two types and three calices (crowns or heads).
I took a chance and cut the concretion with a rock saw if there were interesting features on the inside. There were indeed! In the image above you see at the bottom a cross section through a broken crinoid stem showing the articulated columnals. Above it are sections of crinoid arms (the white and grey spots) each trailing a pair of delicate pinnules (the feeding parts of the arms that carried tube feet). The arms are coming from an intact calyx that is not in the plane of the section.
 In this closer view of the above stem we see the complex anatomy of the crinoid stem. We also see the amazing mineralogy of these specimens in a way we could not from the outside. The light brown matrix is, as we’ve said, the concretion made primarily of siderite (an iron carbonate) and clay. The crinoid columnals, which were originally made of calcite (calcium carbonate), have a silvery metallic material replacing them. This is the iron sulfide mineral marcasite. The white mineral on the inside of the stem on the left is quartz (silicon dioxide). It filled in open spaces inside the stem. To confuse things (nothing is ever easy in this business!) on the right end of the stem marcasite has filled in the cavities instead of quartz.
In this closer view of the above stem we see the complex anatomy of the crinoid stem. We also see the amazing mineralogy of these specimens in a way we could not from the outside. The light brown matrix is, as we’ve said, the concretion made primarily of siderite (an iron carbonate) and clay. The crinoid columnals, which were originally made of calcite (calcium carbonate), have a silvery metallic material replacing them. This is the iron sulfide mineral marcasite. The white mineral on the inside of the stem on the left is quartz (silicon dioxide). It filled in open spaces inside the stem. To confuse things (nothing is ever easy in this business!) on the right end of the stem marcasite has filled in the cavities instead of quartz.
 This view of another stem in cross-section shows a fourth mineral in the system: calcium carbonate. It can be seen as the glassy material in the middle of the structure. It is not the original calcite that made up the columnals. It is instead a later mineral that, like the quartz and marcasite in the previous image, filled in open spaces within the stem. The marcasite, quartz and calcite are thus secondary minerals introduced to the fossil long after its burial. We call these chemical and physical changes to the original mineralogy diagenesis.
This view of another stem in cross-section shows a fourth mineral in the system: calcium carbonate. It can be seen as the glassy material in the middle of the structure. It is not the original calcite that made up the columnals. It is instead a later mineral that, like the quartz and marcasite in the previous image, filled in open spaces within the stem. The marcasite, quartz and calcite are thus secondary minerals introduced to the fossil long after its burial. We call these chemical and physical changes to the original mineralogy diagenesis.
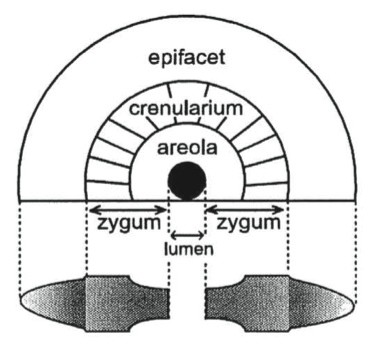 Since this cross-section view of the crinoid stems is surprisingly complicated, here is a diagram from Fearnhead (2008, figure 2). The top is a crinoid columnal looking at its articulating surface. At the bottom is a cross-section. In our crinoids you can easily make out the lumen as a hollow space running through the center of the stems (filled with marcasite, calcite or quartz). The zygum is that portion of the columnal replaced by marcasite.
Since this cross-section view of the crinoid stems is surprisingly complicated, here is a diagram from Fearnhead (2008, figure 2). The top is a crinoid columnal looking at its articulating surface. At the bottom is a cross-section. In our crinoids you can easily make out the lumen as a hollow space running through the center of the stems (filled with marcasite, calcite or quartz). The zygum is that portion of the columnal replaced by marcasite.
Lat week I mentioned that there was a molluscan surprise revealed upon cutting open this concretion. I’ll save that for part III of this series. Same channel next week!
References:
Fearnhead, F.E. 2008. Towards a systematic standard approach to describing fossil crinoids, illustrated by the redescription of a Scottish Silurian Pisocrinus de Koninck. Scripta Geologica 136: 39-61.



Very nice specimen and photography capture.
PL
…just wonder whether the calcite is indeed calcite. It has a slightly greasy lustre. You get various things in some Jurassic concretions that look a bit like calcite but turn out to be Barium or Strontium compounds.
But I expect I just making trouble again…
At least it fizzes!
That is a great set of photos! It’s possible that the marcasite represents the reduced form of other iron oxides/carbonates (hematite and sideritic carbonate) in the surrounding concretion material. If there were enough residual carbon remaining in the body/stem of the crinoid, and since we know there was plenty of sulfur from some source, the carbon may have acted to reduce the oxidation state within the body cavity to allow formation of iron sulfide as opposed to iron carbonate/oxide. It would be interesting to see if a chemical analysis of the surrounding concretion material showed sulfate in the matrix. So, you may be seeing a simple oxidation-reduction “equilibrium” reaction at work due to the presence of the gradient between and oxidizing and reducing environment. It’s analogous to the reaction we see in, and around, Ohio coal beds, where ancient seawater sulfate has been reduced to iron sulfides because of the reducing conditions in the coal.
That makes sense to me as a possibility, Bill. There must be a chain of mineral reactions and transformations here, all beyond my skills to decipher! I am impressed with your mineral chemistry.
Well, the marcasite in the crinoid stems is spectacular, and a lot of near surface (well, anywhere you can get water to flow through rock, so “near surface” is a rather relative term), water-mediated reactions are controlled by oxidation-reduction equilibria. Just like in the classic “roll-front” uranium deposits of the desert SW, or the banded iron formations, we see the oxidation state of the fluid controlling deposition of, in those cases, valuable concentrations of metals (or metal oxides), and it is very common to see metals either oxidized (as carbonates or oxides or sulfates, etc.) change over into sulfides (dominantly) as the fluids which mediate the reaction have their oxidation state controlled by the rocks (and, particularly, the rocks’ composition) through which they flow. Sorry for that rather long sentence.
BTW, there’s an I.S., just in that one specimen, if you have, or have access to, the equipment to analyze, in detail, the chemistry of the minerals as you traverse the surface, particularly at the interfaces between the mineralogic transitions.
That’s a good idea, Bill, the I.S. possibility. I wouldn’t be the advisor, but maybe Meagen Pollock would be interested. We would also have to find additional specimens with provenance. I’m glad these beautiful fossils provoked such an interesting analysis!